Frequently Asked Questions
Answer
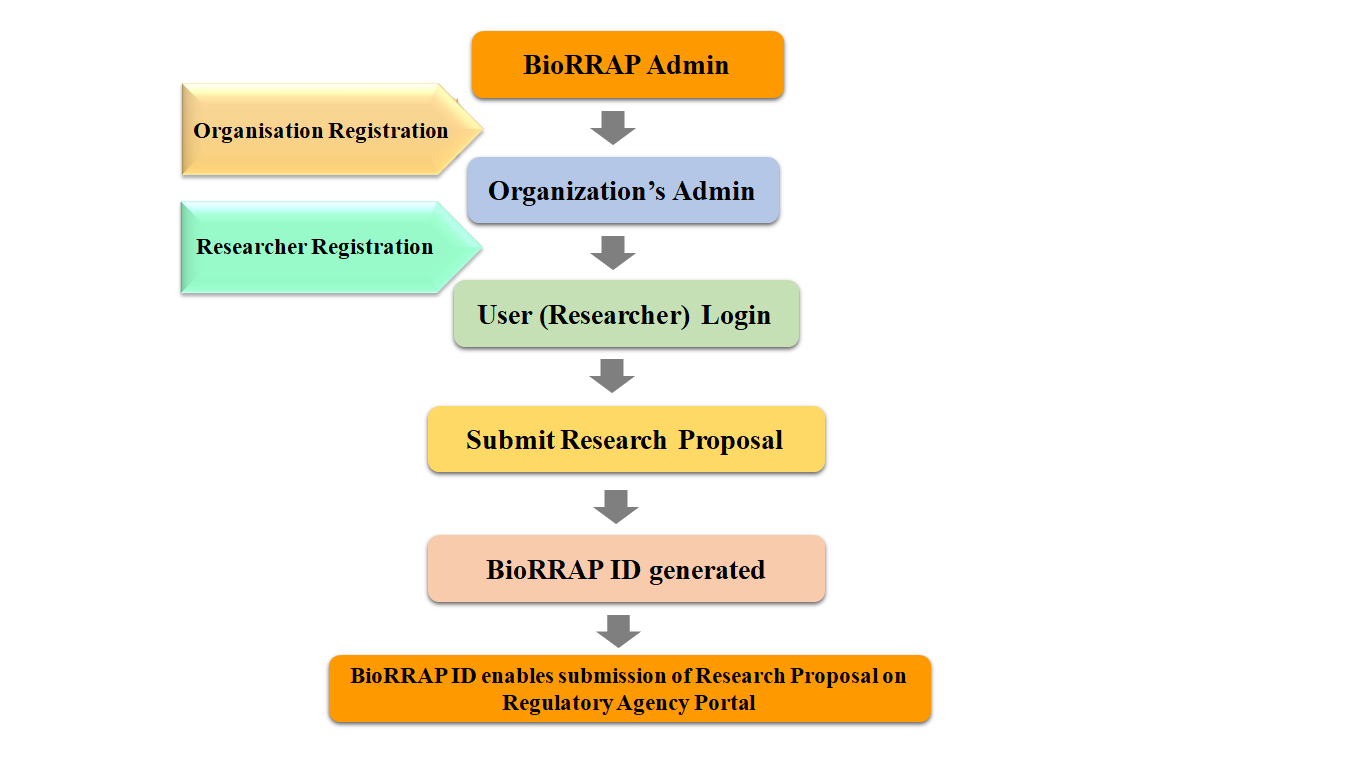
BioRRAP is a centralized mechanism created to facilitate Biological Research Regulatory Approval process. The full Form of BioRRAP is Biological Research Regulatory Approval Portal wherein all researchers and public, private R&D organizations and stakeholders undertaking biological research which require regulatory approval(s) are required to register on this centralized national portal. The BioRRAP ID generated through this portal is linked with the portals of various regulatory agencies.
Answer
When a research proposal is submitted on this portal, a unique BioRRAP ID will be granted for the research which is essential for submission of application to concerned regulatory authority. The researcher would be able to see the status of application submitted to various regulatory agencies using the BioRRAP ID.
Answer
Since each research proposal requires BioRRAP ID for submission on portals of regulatory agencies, it is mandatory to register on BioRRAP. BioRRAP ID will be generated for a research proposal only on submission of the same on BioRRAP portal.
Answer
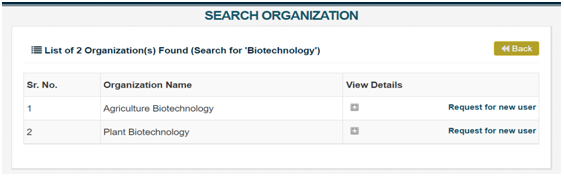
Search your Applicant Agency’s name at “Search Applicant Agency” page of the portal available at http://biorrap.gov.in/Home/SearchOrganization The Applicant Agency registration on BioRRAP is prerequisite for registration of researchers on BioRRAP.
Answer
An Applicant Agency’s admin is enabled to create login credentials for every researcher of the organization. “Add New User” tab is provided at Applicant Agency admin’s dashboard for the same.
Answer
Search your Applicant Agency’s name at “Search Applicant Agency” page. If your Applicant Agency is registered on the BioRRAP, the same will appear in the list of search results. In the row of your Applicant Agency name, click on request “Request for new user” and submit required details. Your request for creating you as a new Researcher/User to enable you to submit research proposal on BioRRAP will be sent to your Applicant Agency’s BioRRAP admin for review and further action. When your Applicant Agency’s BioRRAP admin will approve the request, you will get login credentials on your registered Email. If your Applicant Agency name does not appear in the list of search results; please request your Applicant Agency to register on BioRRAP.
Answer
Login at BioRRAP with login credentials issued to you as a researcher of the Applicant Agency. Thereafter, click on “New Proposal” section to open a new research registration form. Select every option of the form and provide details in each section.
Answer
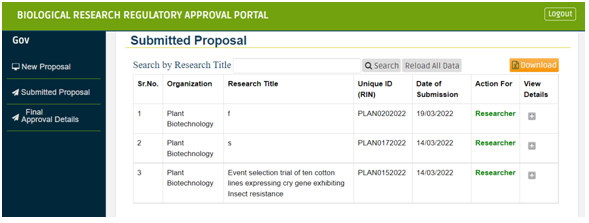
After submission, you can see a list of your Submitted proposals under “Submit Proposal” section. Go on “Action For” and click on “Researcher”. You will get a list of “Regulatory Authority Name” along with “Email ID” and link for their portal under “Action” column.
Answer
The BioRRAP is developed and maintained by the Department of Biotechnology, Ministry of Science & Technology, Government of India.
Answer
If you are a researcher working in the Applicant Agency, contact your Applicant Agency’s admin for BioRRAP.
If you are one of the Applicant Agency’s BioRRAP admin, email details of the issue to biorrap.in@dbt.nic.in
Answer
Your Applicant Agency’s BioRRAP admin may email details of the case to biorrap.in@dbt.nic.in
Answer
Contact your Applicant Agency’s admin for BioRRAP. If issue not resolved, email details of the case to biorrap.in@dbt.nic.in
Answer
Contact your Applicant Agency’s admin for BioRRAP. If issue not resolved, email details of the case to biorrap.in@dbt.nic.in
FAQs on Specific Requirements of Regulatory Approvals
Please also refer the “Guidance Document on Biological Research Regulatory Approvals” for the FAQs below
Answer
The use of r-DNA technology and /or emerging gene technologies for research and development activities is regulated in accordance with Rules, 1989 of the Environment Protection Act 1986. For further details please refer ‘Regulations & Guidelines for Recombinant DNA Research and Biocontainment, 2017’ available at (https://ibkp.dbtindia.gov.in/Content/Rules).
Answer
Please refer “Revised Simplified Procedure/ Guidelines on Import, Export, and Exchange of GE organisms and product thereof for R&D purpose, 2020” available on Indian Biosafety Knowledge Portal (https://ibkp.dbtindia.gov.in/Content/Rules).Import and Export of Regulated items not specified or not covered in the above guidelines will require approval from RCGM. Please also refer the “Guidance Document on Biological Research Regulatory Approvals”.
Answer
Please refer Annexure I and Annexure II of “Handbook for Institutional Biosafety Committees (IBSCs), 2020”, for List of forms/performa and pathways of regulatory approvals for import/export/exchange of GE organisms/hazardous microorganism, respectively, available at https://ibkp.dbtindia.gov.in/Content/Rules.
Answer
As per “Revised Simplified Procedure/ Guidelines on Import, Export, and Exchange of GE organisms and product thereof for R&D purpose, 2020”, exchange of regulated items within India (i.e. Transfer & Receive) for Biopharma Drug Development (R&D) shall not require approval from RCGM. The exchange of materials may take place with the approval from respective IBSCs of organizations involved in transfer/receive before commencing the activity if material to be exchanged falls under the provisions of aforementioned simplified procedure.
Answer
Each investigator handling recombinant DNA materials for research must register their Applicant Agency and IBSC on the IBKP Portal with details of bio-containment facilities available at their Applicant Agency and the kind of micro-organisms being handled at their premises. Further, for the guidance on regulatory approvals, kindly refer “Revised Simplified Procedure/ Guidelines on Import, Export, and Exchange of GE organisms and product thereof for R&D purpose, 2020” available at IBKP Portal (https://ibkp.dbtindia.gov.in/Content/Rules).
Answer
Please refer “List of infective Microorganisms corresponding to different Risk Groups, 2021” available athttps://ibkp.dbtindia.gov.in/Content/Rules
Answer
Experiments on RG-1 microorganisms fall under category I experiments. For information on regulatory approvals, kindly refer ‘Regulations & Guidelines for Recombinant DNA Research and Biocontainment, 2017’ (pg no. 27-42) available at (https://ibkp.dbtindia.gov.in/Content/Rules).
Answer
For regulatory permissions required to use pathogenic microorganism for research and development, please refer ‘Regulations & Guidelines for Recombinant DNA Research and Biocontainment, 2017’ available at (https://ibkp.dbtindia.gov.in/Content/Rules).
Answer
The researchers are required to get approval on the animal experiment protocols from Institutional Animal Ethical Committee under PCA Act 1960 and Breeding of and Experimentation (Control and Supervision) Rules 1998, as amended in 2001 and 2006. Each Institution undertaking such work has to form an Institutional Animal Ethics Committee for control and supervision of experiments on animals performed in the Institute.
Further, if the research proposal on the animal experiments involves any rDNA work/HMO/LMO/GMO then IBSC approval followed by RCGM approval in form C1 at IBKP portal is required.
For conducting preclinical toxicity studies on recombinant product, IBSC approval followed by RCGM approval to in Form C3 at IBKP Portal is required.
Answer
Depending on the Risk group and/or category the biological material, you may require to take regulatory approvals. For further details please refer ‘Regulations & Guidelines for Recombinant DNA Research and Biocontainment, 2017’ available at (https://ibkp.dbtindia.gov.in/Content/Rules).
Answer
For experimentation on large animals, CPCSEA approval is required based on recommendations of IAEC (Institutional Animal Ethics Committee).
Answer
Please refer “Regulations & Guidelines for Recombinant DNA Research and Biocontainment, 2017” available at (https://ibkp.dbtindia.gov.in/Content/Rules).
Answer
Certification of BSL-3 Laboratories is mandatory for all organizations handling hazardous microorganisms for research and development purpose w.e.f. 01.04.2021. Further, all new BSL-3 facilities will undergo Certification prior to commencement of facility operation.
Answer
All organizations involved in research, development and handling of the genetically engineered (GE) microorganism and non-GE hazardous microorganisms need to comply with the “Guidelines for the Establishment of Containment Facilities: BSL-2 and BSL-3 and Certification of BSL-3 facility, 2020”. The application for Certification of BSL-3 Facility is submitted to RCGM Secretariat at rcgm.dbt@nic.in for consideration of the ‘Interministerial committee for Certification of BSL-3 Facility’ and RCGM.
Answer
Institutional Biosafety Committees (IBSC) is required to be constituted by every Applicant Agency working on rDNA technology or hazardous microorganisms. IBSC is responsible for ensuring biosafety at R&D level in the laboratory and containment conditions. For more details, please refer “Handbook for Institutional Biosafety Committees (IBSCs), 2020” available at IBKP Portal (https://ibkp.dbtindia.gov.in/Content/Rules).
Answer
Constitution of an IBSC and its registration in Department of Biotechnology (DBT) through Indian Biosafety Knowledge Portal(IBKP) is mandatory in India for every Applicant Agency undertaking R & D activities using GE organisms/ cells and hazardous microorganisms in accordance with the Rules 1989 of the EPA, 1986. For details, please refer “Handbook for Institutional Biosafety Committees (IBSCs), 2020” page 12-24, available at on IBKP portal(https://ibkp.dbtindia.gov.in/Content/Rules).
Answer
Apart from adhering to the ICMR guidelines, If the research and development activities on human biosamples involves any rDNA work/HMOs/GMOs/LMOs, subsequent approvals is required from IBSC/RCGM depending on the category of experiments as specified in the “Regulations & Guidelines for Recombinant DNA Research and Biocontainment, 2017”.
Answer
Applicant can procure the infectious biosamples/biospecimen from Biorepositories. For details, please refer “SOPs for exchange of infectious biosamples/biospecimen from Biorepsitory, 2021” available at https://ibkp.dbtindia.gov.in/Content/Rules
Answer
SCOMET is an acronym for Special Chemicals, Organisms, Materials, Equipment and Technologies. This list contains goods which are considered as dual-use items, i.e., goods, technology, chemicals, organisms etc.
Answer
The SCOMET items are classified under 9 distinct categories:
Category 0: Nuclear materials, nuclear related other materials, equipment and technology
Category 1: Toxic chemical agents and other chemicals
Category 2: Micro-organisms, toxins
Category 3: Materials, Materials Processing Equipment and related technologies
Category 4: Nuclear-related other equipment and technology, not controlled under Category0
Category 5: Aerospace systems, equipment including production and test equipment, relatedtechnology and specially designed components and accessories thereof
Category 6: Munitions list
Category 7: Reserved
Category 8: Special Materials and Related Equipment, Material Processing, Electronics,Computers, Telecommunications, Information Security, Sensors and Lasers, Navigation andAvionics, Marine, Aerospace and Propulsion.
Answer
The list of ‘SCOMET’ items is available in the Import, Export & SCOMET Policy in the Regulatory Updates section in DGFT website at http://dgft.gov.in/CP/.
Answer
No
Answer
For guidance on regulatory approvals of import/export/exchange within the country of Model organisms, kindly refer section 1.5 of Table B of Annexure 1 of “Revised Simplified Procedure/ Guidelines on Import, Export, and Exchange of GE organisms and product thereof for R&D purpose, 2020” available on Indian Biosafety Knowledge Portal (https://ibkp.dbtindia.gov.in/Content/Rules).
